Collaboration between Project 4 (CML) and Novartis
The CML Group within the European Leukemia Network agreed on a long term Collaboration with Novartis to improve Understanding and
Treatment of chronic myeloid Leukemia
Authors: S. Saußele, R. Hehlmann, Information Letter No.4, October 2007
The collaboration
On 28. June 2007 the European LeukemiaNet (EL N) and Novartis launched a collaboration committed to improving understanding and treatment of chronic myeloid leukemia (CML-alliance).
The collaboration was initiated by the signature of a Scientific Collaboration Agreement between the CML-ELN group represented by the University of Heidelberg, Germany, and Novartis, Switzerland (see Fig. 1).
The objectives of the CML alliance with Novartis are to:
- Increase understanding of the epidemiology of CML, its treatment and real life outcomes by collecting baseline-, treatment- and outcome data of representative samples of CML patients of major European countries
- Develop and validate a comprehensive prognostic model which allows to optimize individual treatment choices
- Evaluate the implementation of the EL N recommendations for CML management (Baccarani et al., Blood 2006;108:1809-20.) as reference; encourage uptake of EL N recommendations for the management of CML and measure clinical benefit of their implementation
- Promote quality controlled molecular monitoring using standardized RQ-PCR technologies and an international definition of major molecular response
- Assess impact of drug monitoring, pharmacokinetics and patients’ compliance on the course of CML
- Foster continued medical education and spread of excellence
- Provide a platform for the expedited evaluation of new treatments
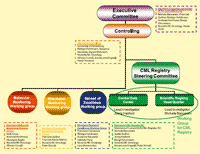
To reach these goals four subprojects are created (see Fig. 2).
Figure 2. Organizational structure of the CML-Alliance: The executive committee directs the Alliance scientifically. The management board is responsible for the management and controlling of the whole project. The Registry project is led by a Steering Committee with two central units (Scientific Registry Headquarter in Bologna and Central Data Center in Munich). The other three subprojects are led by chairs as indicated.
Subproject Registry
A key activity of the collaboration is to expand the already existing CML Registry in collaboration with workpackage 17. The major idea is to collect baseline-, treatment- and outcome data of representative samples of CML patients in European countries by implementing and enlarging the current EL N-CML Registry and its related subregistries. Its activities will be enhanced through the collaboration agreement with Novartis. Due to the limited funding by the European Commission (EC) the registry, at the moment, enrolls only study patients. With the additional financial support more countries and population based data can be included.
This will lead to a real-world pan-European picture of the incidence, distribution and control of CML and will provide a clear picture of the current management of CML.
Subproject Molecular Monitoring
This subproject strengthens the registry by providing quality controlled outcome data and improves the availability of molecular monitoring for CML patients by reshaping existing European infrastructure. The distribution of internationally standardized RQ-PCR analyses for BCR-ABL quantification and for detection of BCR-ABL mutations will facilitate the investigation of response and acquired resistance to existing treatments, helping to inform on therapeutic choices for these patients.
Subproject Pharmacological Monitoring
This initiative will expand the availability of imatinib monitoring to a European level in order to optimise therapy for a greater number of patients. This will be achieved by providing a Europewide monitoring service free of charge (sample transportation and dosing) for a period of 3 years. During this period, a database will be constructed to i) verify the pertinence of the therapeutic threshold by following patients with imatinib levels < 1000 ng/ml and ii) to define a toxic concentration. Potential dosing laboratories will be selected across Europe (one or two for each participating country) and a dosing service established in the respective countries by collaboration with the Bordeaux center which will also manage a centralised quality control system.
Subproject Spread of Excellence (SoE)
The objective of this subproject is to raise awareness of the CML alliance with the definite goal to foster EL Nactivities, promote implementation of EL N management recommendations, improve understanding and treatment of CML and enhance outcome of CML across Europe and globally. The SoE project will support all activities that promote realization of a European CML registry and of the other subprojects
of the CML-alliance. The activities for the spread of excellence can be broken down to various scientific, organizational, educational and PR activities.
During a start symposium in Heidelberg in September 2007 milestones and activities of all four subprojects were discussed and planned. At a
booth of the EL N at the ASH congress in December 2007, this registry project will be further introduced and educational material will be also available. A first progress report is planned for the annual EL N-Symposium end of January 2008